Vinyl Alcohol Resonance Structures

It is not a precursor to polyvinyl alcohol synthesis.
Vinyl alcohol resonance structures. Vinyl acetate c4h6o2 cid 7904 structure chemical names physical and chemical properties classification patents literature biological activities safety. Begingroup to see a similar substance that can undergo resonance look at phenol. In both resonance structures the central o atom is bonded to the two outer o atoms and has one nonbonding pair. Lewis structures of acetaldehyde ethylene oxide and vinyl alcohol.
Their thermal behaviors were investigated by differential scanning calorimetry and thermogravimetric analysis tga. C 2x8 16 h 4x2 8 o 1x8 8 total 32. An electron donating group edg or electron releasing group erg z in structural formulas is an atom or functional group that donates some of its electron density into a. C 2x4 8 h 4x1 4 o 1x6 6 total 18 step2.
Find valence e in all atoms. Lewis structure of c 2 h 4 o. Because of resonance the bonds between the central o atom and the outer o atoms are of equal length. Endgroup alaskaron dec 17 15 at 4 18.
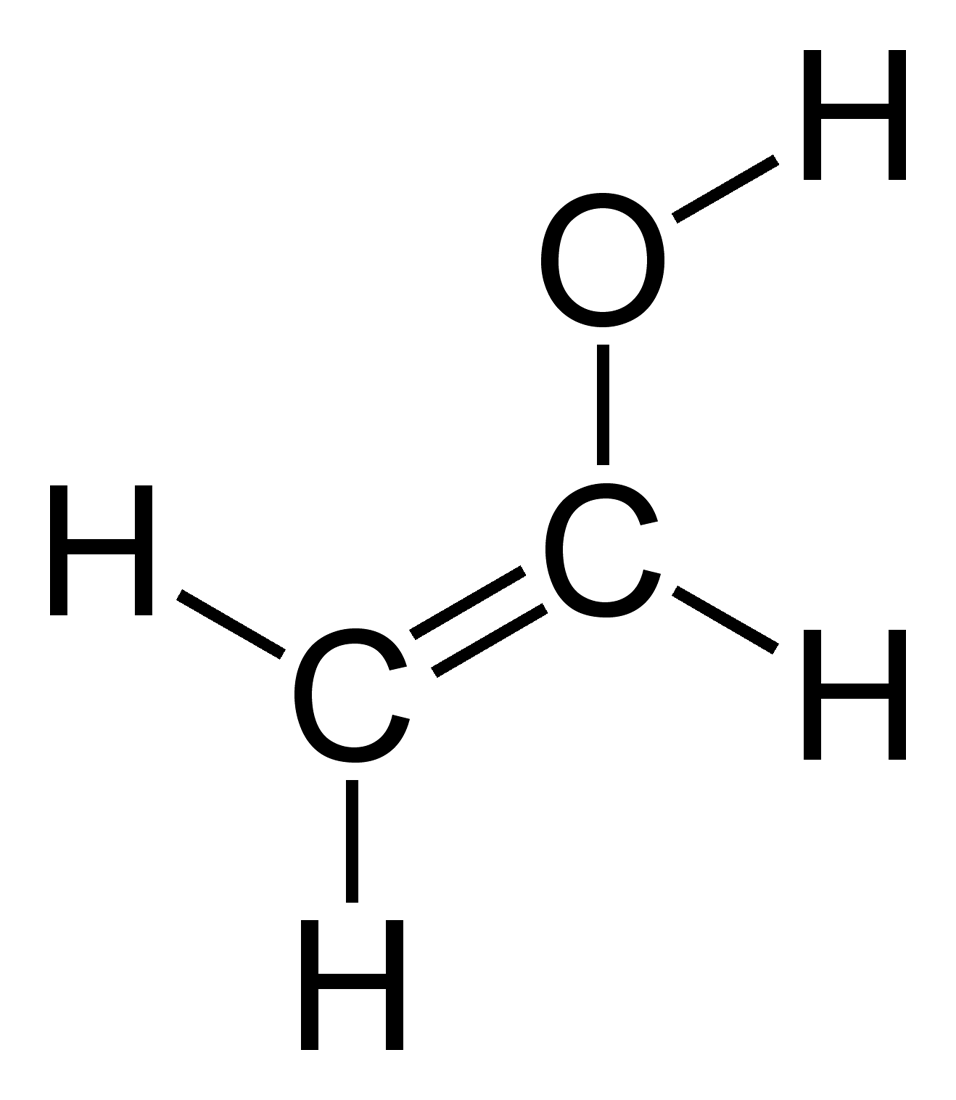
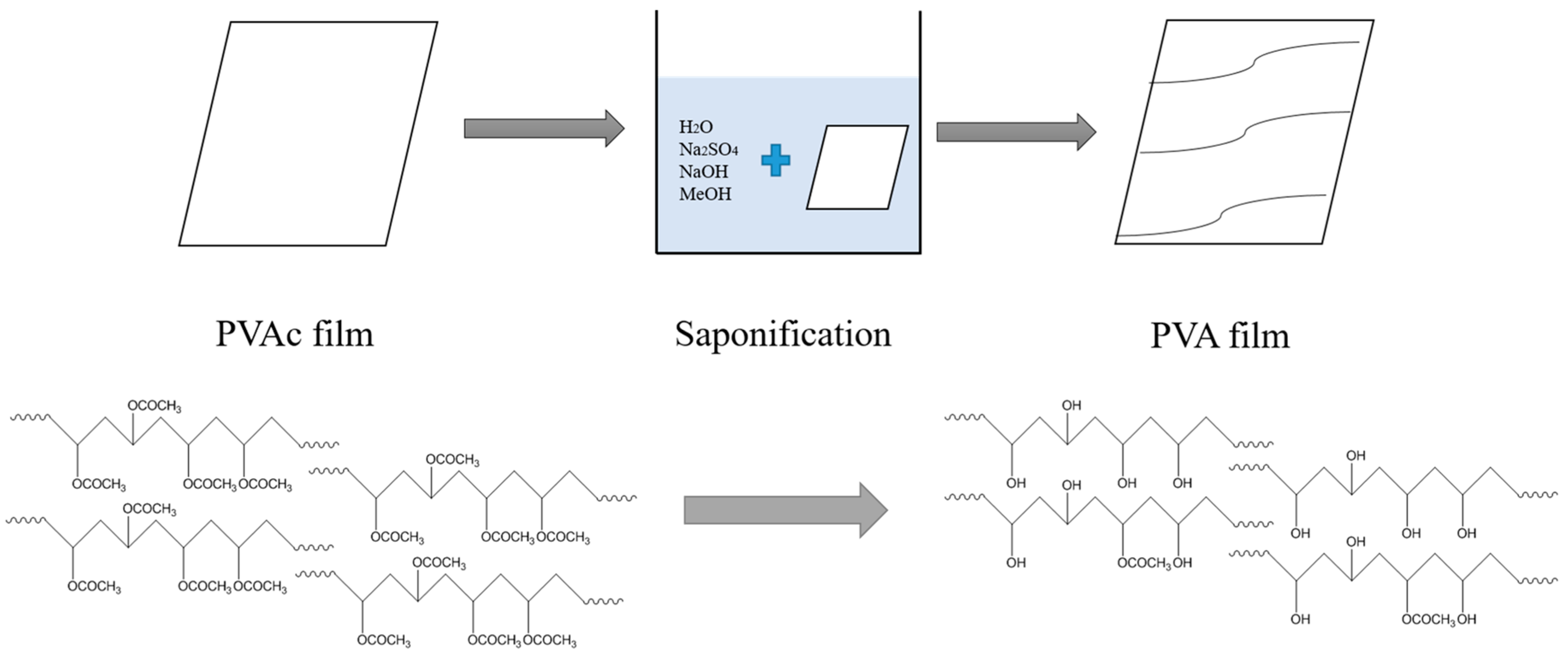
In an electrophilic aromatic substitution reaction existing substituent groups on the aromatic ring influence the overall reaction rate or have a directing effect on positional isomer of the products that are formed. Vinyl alcohol also called ethenol iupac name is the simplest enol. The structure of a poly vinyl alcohol pva iodine complex was investigated by a resonance raman spectroscopy wide angle x ray diffraction small angle x ray. Vinyl alcohol can be formed by the pyrolytic elimination.
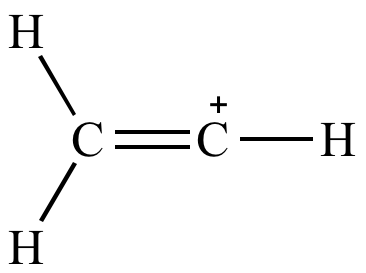
With the formula c h 2 choh it is a labile compound that converts to acetaldehyde. Sometimes resonance structures are not equivalent and it is important to determine which one s best describe the actual bonding. Alcohols like vinyl alcohol really overdo it they isomerize to aldehydes. Remember that a double bond counts as a single electron domain.
The different resonance structures of the carbonate ion co 3 2 are illustrated above the delocalization of electrons is described via fractional bonds which are denoted by dotted lines and fractional charges in a resonance hybrid. A careful analysis of the 50 mhz 13c nuclear magnetic resonance n m r spectrum of fully hydrolysed poly vinyl alcohol and a critical comparison wi.