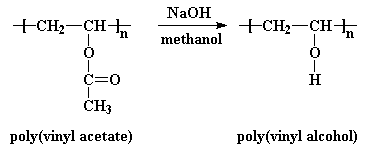Synthesis Of Polyvinyl Alcohol From Vinyl Acetate

Poly vinyl acetate pvac chains of a low polydispersity mw mn 1 1 1 2 have been prepared by cobalt mediated radical polymerization of vinyl acetate vac.
Synthesis of polyvinyl alcohol from vinyl acetate. The glass transition temperature of polyvinyl acetate is between 30 and 45 c depending on the molecular weight. It is commonly supplied as beads or as solutions in water. Poly vinyl alcohol pvoh pva or pval is a water soluble synthetic polymer it has the idealized formula ch 2 ch oh n it is used in papermaking textile warp sizing as a thickener and emulsion stabilizer in pvac adhesive formulations and a variety of coatings. Polyvinyl alcohol pva a colourless water soluble synthetic resin employed principally in the treating of textiles and paper.
It is colourless white and odorless. Pva is unique among polymers chemical compounds made up of large multiple unit molecules in that it is not built up in polymerization reactions from single unit precursor molecules known as monomers. Synthesis of poly vinyl alcohol silica gel polymer hybrids by in situ hydrolysis method ryo tamaki and yoshiki chujo department of polymer chemistry graduate school of engineering kyoto university yoshida sakyo ku. The repeating unit of vinyl alcohol is not used as the starting material because it is unstable and cannot be isolated.
It is easily tautomerized into acetaldehyde which is a very stable resonance structure of vinyl alcohol see figure 1. The degree of polymerization of polyvinyl acetate is typically 100 to 5000 while its ester groups are sensitive to base hydrolysis and slowly convert pvac into polyvinyl alcohol and acetic acid. Synthesis of pva might be accomplished by many methods. Polyvinyl alcohol pva is produced through the hydrolysis of polyvinyl acetate.
The easiest method would be to polymerize vinyl alcohol. Instead pva is made by dissolving another polymer polyvinyl. Unfortunately there is a problem with this approach vinyl alcohol is a chemical substance that is very unstable. The polymer is considered a nonhazardous material according to the american standard for precautionary labeling of hazardous industrial chemicals.
Poly vinyl alcohol undergoes chemical reactions in a manner similar to other secondary polyhydroxy alcohols and is fully biodegradable. Emulsion polymerization of styrene st and vinyl acetate vac in the presence of conventional polyvinyl alcohol pva pva modified with a terminal alkyl group or pva modified with a terminal thiol group hs pva was compared.