Substitution At Vinylic Position Of Alkene

Vinyl iodides are versatile molecules that serve as important building blocks and precursors in organic synthesis.
Substitution at vinylic position of alkene. At higher temperatures however the addition reaction is increasingly superseded by the allylic substitution. On or bonded to the carbon of an alkene. They are commonly used in carbon carbon forming reactions in transition metal catalyzed cross coupling reactions such as stille reaction heck. As they are unreactive towards carbonyl functions these reagents should be more suitable than the corresponding organomagnesium compounds in reactions involving alkenes having such a function in their structure.
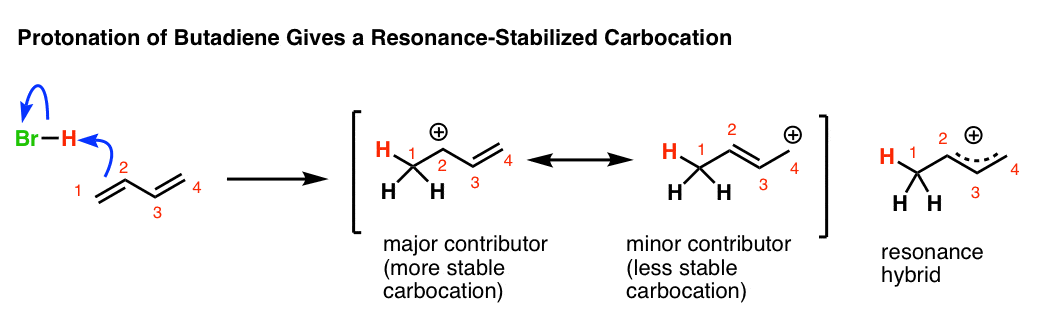
Lithium and magnesium organocuprates can thus be used as arylating agents in the pd promoted vinylic hydrogen substitution of alkenes. Generally at room temperature chlorine and bromine are added to the double bonds of alkenes. Return to glossary index. The c h bond in allylic position most easily cleaved homolytically since the allyl radical is resonance stabilized.
The alkylic allylic and vinylic hydrogens. Only the hydrogen at the central carbon is vinylic. The product is an allylic halide halogen on carbon next to double bond carbons which is acquired through a radical chain mechanism. The specific characteristic of allylic systems is reflected in their chemical reactions.
The name is also used for any compound containing that group namely r ch ch 2 where r is any other group of atoms. Lewis structure of vinyl chloride a vinyl ic halide. In a molecule the position next to an alkene. This molecule has four vinyl ic positions each marked with.
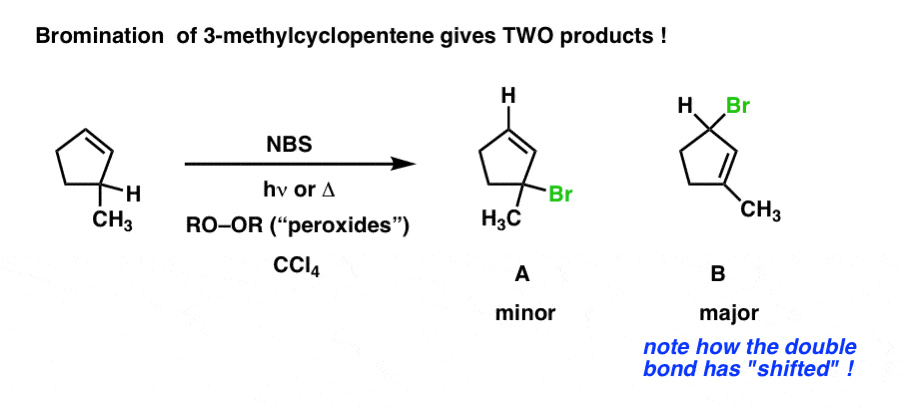
A monosubstituted alkene is an alkene in which the doubly bonded carbons are bonded to only one carbon excluding each other. The allylic positions are labeled with asterisks. However when the halogen concentration is low enough alkenes containing allylic hydrogens undergo substitution at the allylic position rather than addition at the double bond. In organic chemistry a vinyl iodide also known as an iodoalkene functional group is an alkene with one or more iodide substituents.
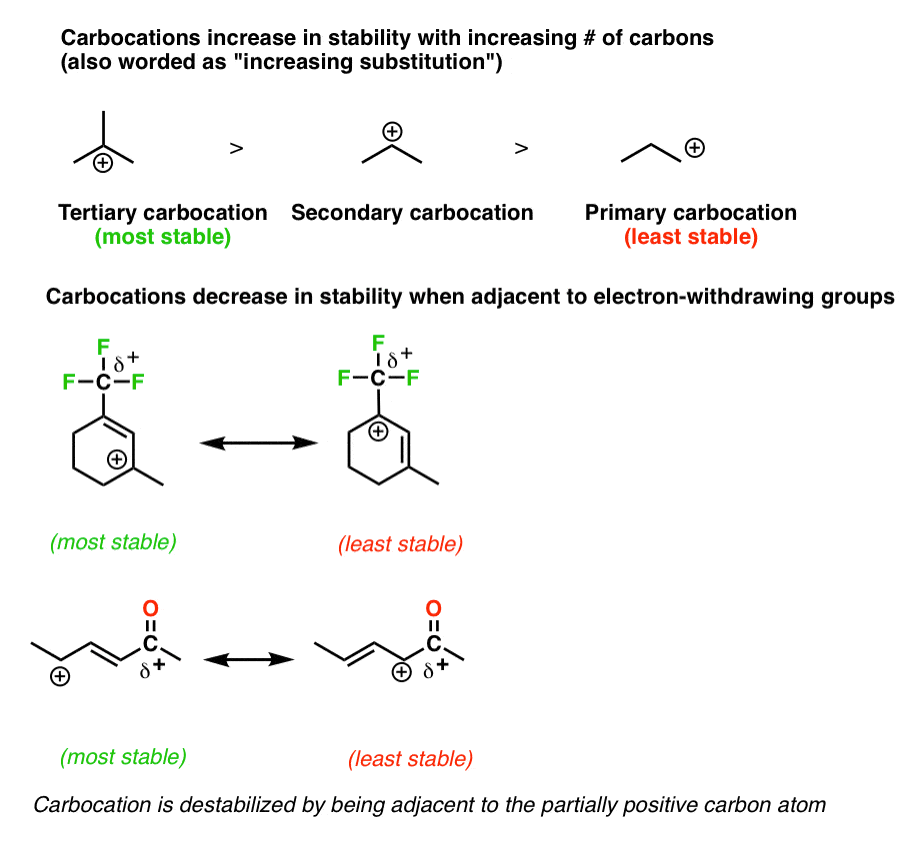
The allylic cyclohexenyl radical is about 40 kj mol more stable than the alkylic cyclohexyl radical is. See also disubstituted alkene trisubstituted alkene tetrasubstituted alkene. In chemistry vinyl or ethenyl abbreviated as vi is the functional group with the formula c h ch 2 it is the ethylene iupac ethene molecule h 2 c ch 2 with one fewer hydrogen atom. Cyclohexene contains three types of hydrogen atoms.
Journal of organometallic chemistry 2006 691 11 2346 2357.