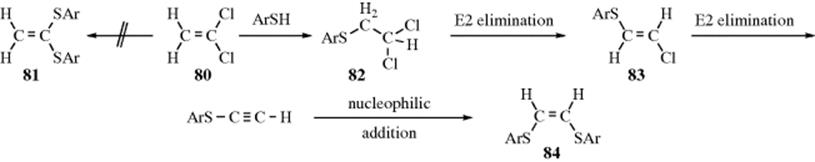
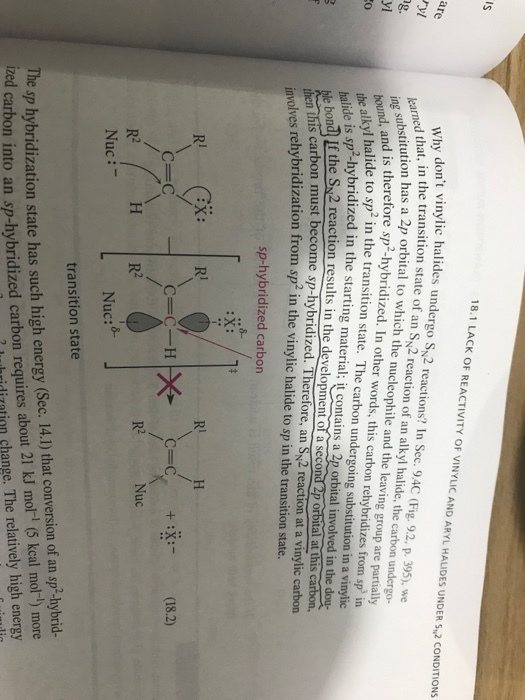
Substitution At Vinylic Carbon
Hughes and sir christopher ingold studied nucleophilic substitution reactions of alkyl halides and related compounds.
Substitution at vinylic carbon. Reactions of morpholine with 4 halocoumarins 1 3 halocoumarins 3 and 2 halo l 4 naphthoquinones 8 yield two different products one where halogen is replaced by a nucleophile at the same carbon and the other where the nucleophile is attached to the vicinal carbon away from that bearing the halogen. The product is an allylic halide halogen on carbon next to double bond carbons which is acquired through a radical chain mechanism. Vinylic chlorides and bromides constitute a diverse class of marine natural products. As the table below shows the dissociation energy for the allylic c h bond is lower than the dissociation energies for the c h bonds at the vinylic and alkylic positions.
1 for such a concerted bimolecular nucleophilic substitution at a vinylic sp 2 carbon are proposed two possible mechanisms namely in plane. Factors conducive to the energetic preference for the in plane sn2 pathway. Journal of the american chemical society 2000 122 10 2294 2299. Vinylic halides natural occurrence.
Nucleophilic substitution at unactivated vinylic carbon. Why substitution of allylic hydrogens. Nucleophilic substitution at a vinylic carbon is difficult under normal condition and is extremely slow compared to substitution at saturated carbon. Concerted nucleophilic substitution at an sp 3 carbon typically bimolecular nucleophilic substitution s n 2 reaction is one of the most fundamental reactions in organic chemistry giving a substitution product with inversion of the configuration.
It is considered that reactions proceeds through nucleophilic vinylic substitution. The two main mechanisms are the s n 1 reaction and the s n 2 reaction. Nucleophilic substitution at a vinylic carbon concerned with the nucleophilic substitution at unsaturated carbon such as vinylchloride ch chcl. In line formulas such as the following a carbon atom is assumed to be at every intersection of two lines and at the end.
In 1935 edward d.