Rug Facts Labels List Information In The Following Order

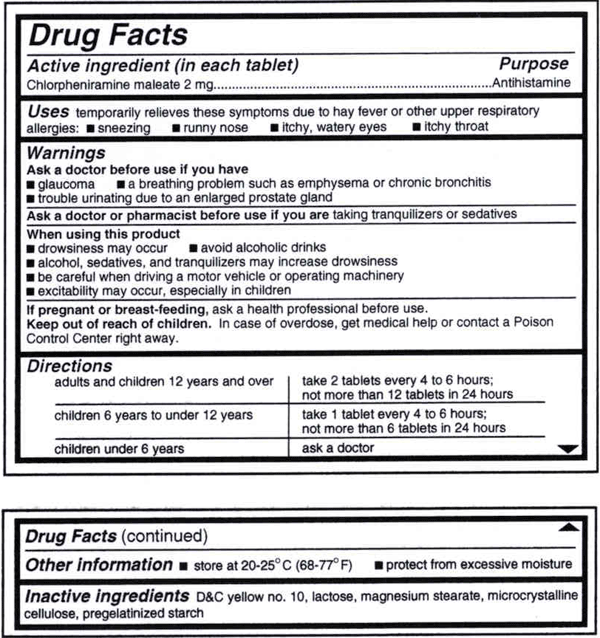
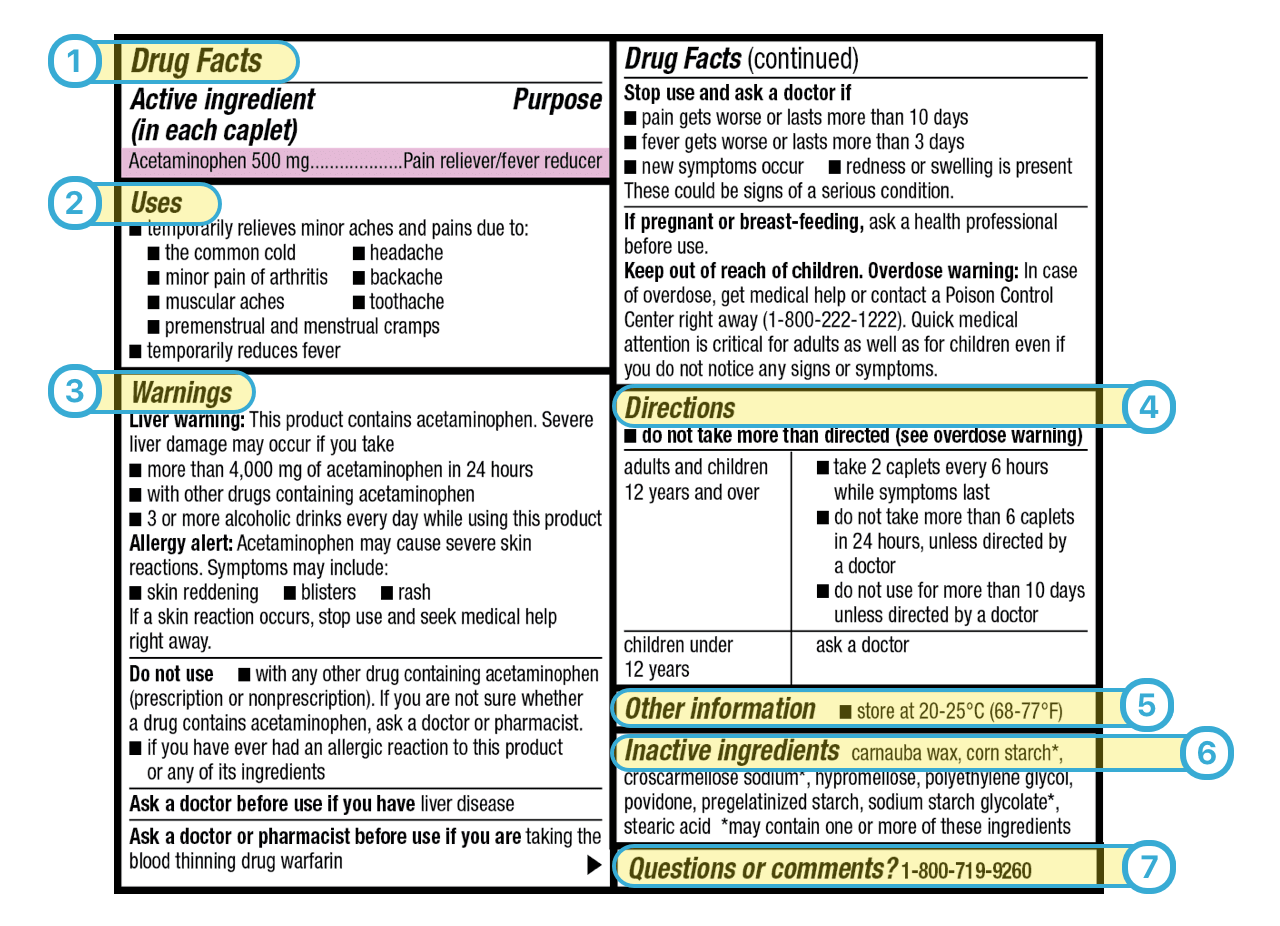
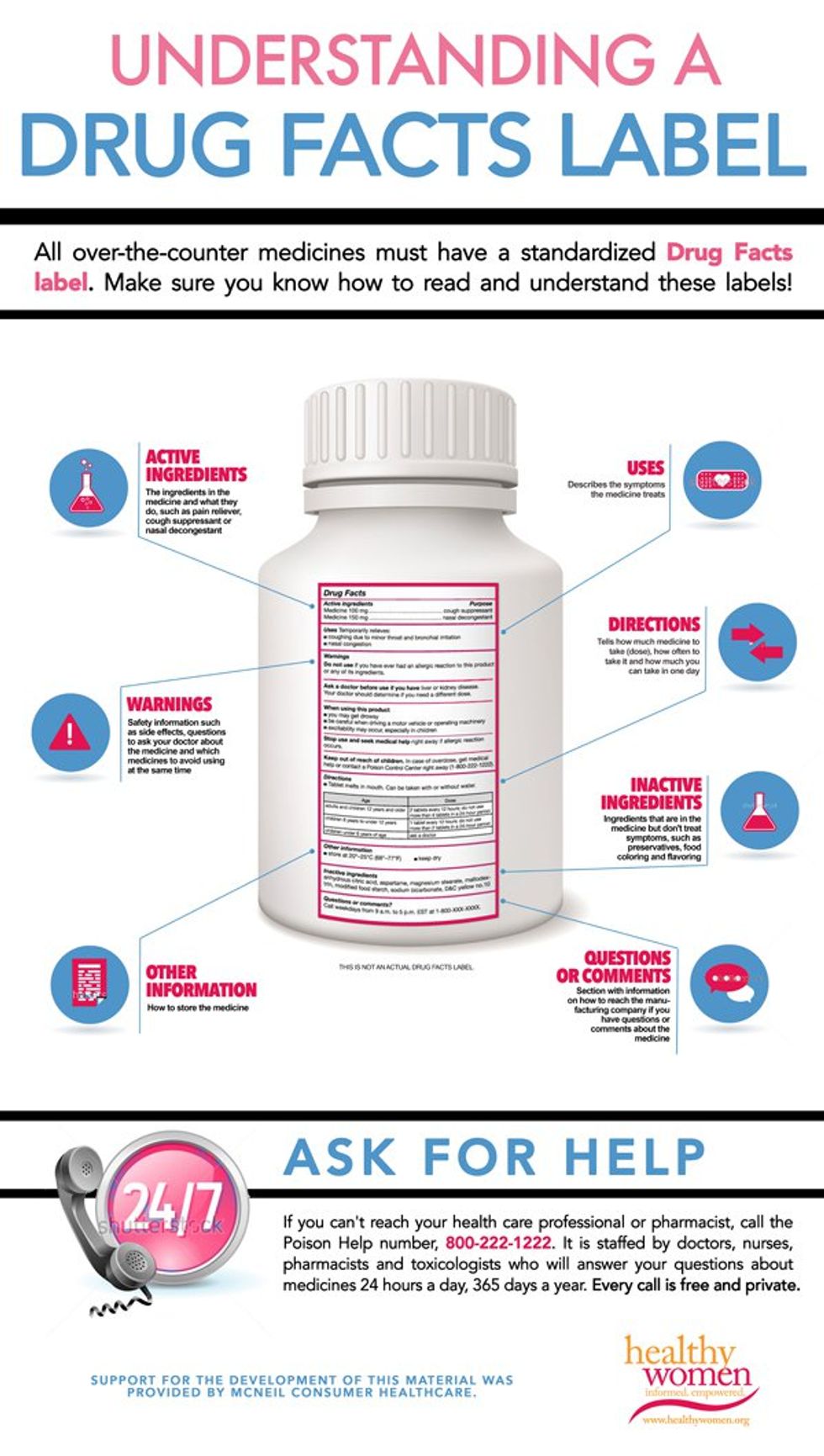
Drug facts labels list information in the following order.
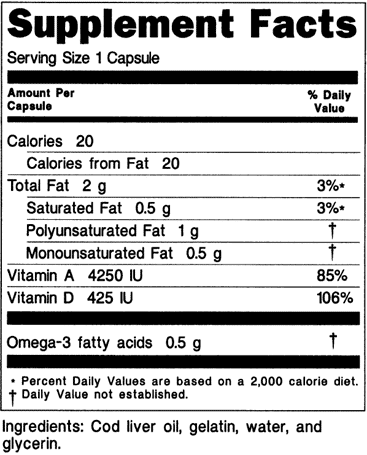
Rug facts labels list information in the following order. Fda is requiring changes to the nutrition facts label based on updated scientific information new nutrition research and input from the public. All over the counter otc medicines contain a drug facts label on their outer package that tells you how when and how often to use a medicine. This is the first major update to the label in. The drug facts label is only required for otc drugs and it is not used for dietary supplements such as vitamins minerals and herbal remedies.
This is a copy of a typical drug facts label. Please be aware of the following when using information from this web site. It also provides important information that is always presented in the same format. Fda has provided for a flexible implementation schedule.
The supplement facts panel on the labels of intermediate sized packages must use type size no smaller than 6 point except that type no smaller than 4 5 point may be used on packages that have. How to read a drug facts label. See 21 cfr part 207 the drug labeling and other information has been. Other information that is required by the fda in a monograph or product approval is listed below the directions.
Always read the label the fda requires the labels on all otc medicines to have the information listed in the same order to be arranged in a simple eye catching consistent style and to contain words. Patterned after the nutrition facts food label the drug facts label uses simple language and an easy to read format to help people compare and select otc medicines and follow dosage instructions. Asked sep 17 2015 in health biomechanics by pitbull a directions warnings active ingredients uses purpose. 17 test your knowledge true or false.
The last required information category is an alphabetical listing of inactive ingredients. Promotional information may not appear in the other information section or elsewhere within the drug facts portion of the label.